What is HOCL?

History of Hypochlorous Acid
Hypochlorous acid was identified in 1834 by a French chemist, Antoine Jérôme Balard and was used as an antiseptic for traumatic wounds in World Wars I and II. Environmental sanitation applications were later developed to aid gangrene, diphtheria, and scarlet fever. Decades later came the discovery that HOCL is naturally formed within human neutrophils. Its biocidal properties are very powerful, and its benefits are endless.
HOCL is documented with numerous studies of how it has excelled in professional fields such as ophthalmology, dentistry, veterinary, and wound care. HOCL has proven to be highly effective against viruses such as SARS-CoV 2, E. coli, Salmonella, Listeria, MRSA, Norovirus, H1N1, Staph/VRE, Pseudomonas, and all Avian Flus, even at lower concentrations. It is also recognized and regulated by the FDA, CDC, EPA, NSF, and USDA.
How is HOCL Made?
Hypochlorous acid can be produced in a couple of ways: one is by combining water (H2O) and salt (NaCI) to make a salt water brine which then passes through an electrolyzed chamber and is often referred to as Electrolyzed Water or EOW. This approach is widely used, however this can require a setup of some hefty and expensive equipment.

How is the Powder-Form of HOCL Made?
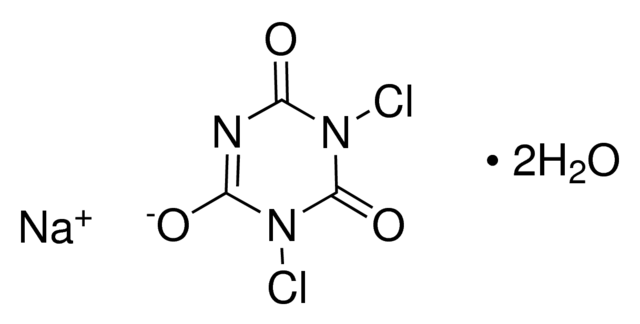
Sodium dichloroisocyanurate dihydrate, or NaDCC (anhydrous: CAS no. 2893-78-9; dihydrate: CAS no. 51580-86-0) is a solid chlorine bleaching agent generally used as an alternative to sodium hypochlorite (bleach). The granules are generally made when NaDCC and a dilute solution of copper (II) sulfate are mixed, producing an intense lilac precipitate of the complex salt sodium copper dichloroisocyanurate. The reactions between dichloroisocyanurate salts (Na, K, Li, Ba, Ca) and transition metal salts (Ni, Cu, Cd) are described in patent US 3'055'889. The overall reaction is: CuSO4 + 4 Na(C3N3O3Cl2) → Na2[Cu(C3N3O3Cl2)4] + Na2SO4 Although the bleaching agent in most chlorine-based bleach is sodium hypochlorite, the sodium salt of dichloroisocyanuric acid, sodium dichloroisocyanurate, is the active ingredient in many commercial disinfectant bactericides, algicides, and cleaning agents. When our powder is mixed in an appropriate water dilution, it produces a chemical reaction, among a variety of chlorinated and non-chlorinated isocyanurates, and free available chlorine (FAC) in the form of hypochlorous acid (HOCL). Hypochlorous Acid is widely used for treating potable water, wastewater, water in swimming pools and spas, and many industrial water systems.
HOCL is the most effective oxidant in the chlorine family, which by nature, gives it a light chlorinated smell that quickly evaporates once bacteria is deactivated. When diluted with H2O, our concentrate results in a chemical reaction that creates hypochlorous acid, a stronger oxidant than chlorine and household bleach under standard conditions.

We pay attention to the pH levels in what we eat, drink, and put on our skin. It is an important quantity that reflects the chemical conditions of a solution. The pH can also control microbial activity and the behavior of chemicals. To ensure that your solution is at it's highest efficacy, test it once a month with pH strips to check that the pH remains between 5-7, where HOCL is most present.

HOCL has a neutral pH of 5-7, making it a weak acid. On the other hand, hypochlorite bleach (OCl-) has a pH level of 12-14, which is hazardous to your eyes, lungs and skin.
Bleach, or hypochlorite (OCl-) is negatively charged, whereas Hypochlorous acid (HOCL) has a neutral charge. Disinfectants and pathogens interact like magnets, attracting or repelling each other. Bacteria and hypochlorite (OCl-) repel each other because they are both negatively charged.

Hypochlorous acid's neutral charge does not repel bacteria, but in fact, attracts them. Bacteria walls are easily penetrated, resulting in quickly killing the cells.
For a thorough review of Hypochlorous Acid, click the link below:
Science Direct: Hypochlorous Acid Review
